Abstract
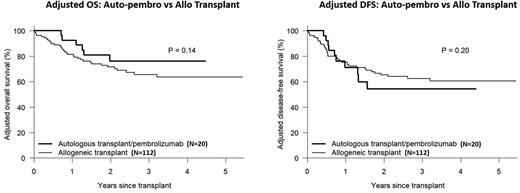
Allogeneic transplant (allo) remains the best post-remission therapy for non-favorable risk acute myeloid leukemia (AML) patients, secondary to the potent graft-versus-leukemia (GVL) effect of donor immune cells. Unfortunately, some patients may be ineligible for allogeneic transplant due to psychosocial barriers (e.g. lack of appropriate caregiver support) or unavailability of a suitable donor. Inhibition of the programmed cell death protein-1 (PD-1) pathway is an effective immunotherapeutic anti-cancer strategy for a number of malignancies. We hypothesized that PD-1 blockade following autologous transplant might simulate an allogeneic GVL effect and represent an alternative post-remission strategy in patients with non-favorable risk AML. To this end, we initiated a phase II study whereby AML patients deemed ineligible for allogeneic transplant received a high dose preparative regimen consisting of fludarabine on day -4, -3, -2 (30mg/m2/d) and melphalan on day -1 (180mg/m2 for age ≤60, 140mg/m2 for age >60), followed by autologous stem cell transplantation and post-transplant pembrolizumab starting day +1 (200mg/dose q3weeks x 8 doses) (auto-pembro). Eligible patients were AML patients beyond CR1 or non-favorable CR1 patients, including c-kit-mutated core-binding factor and FLT3-ITD+/NPM1+ AML. Of 32 patients consented/screened for the study, 20 received protocol-directed therapy from January 2017 to July 2021. Reasons for not receiving transplant were mobilization failure [6], failure to achieve CR [2], patient refusal [2], compliance issues [1] and insurance issues [1]. Baseline patient characteristics included a median (range) age of 64 (26-75) years, KPS <80 in 30%, HCT-CI ≥3 in 85% and non-Hispanic white in 45%. Disease characteristics included ELN 2017 favorable, intermediate and adverse risk in 20%, 40% and 40%, disease-risk index low, intermediate and high in 10%, 75% and 15%, secondary/treatment-related in 20%, CR1 status in 80%, and pre-BMT measurable residual disease (MRD) in 30%. Treatment was well tolerated in only 1 non-relapse death. Infections included 5 patients with bacterial infections, 2 with CMV viremia, 1 with localized VZV and 1 patient with HHV6 reactivation. Grade 3 non-hematologic toxicities included febrile neutropenia (30%), nausea/vomiting (25%), diarrhea (25%), hypertension (20%) and stomatitis/esophagitis (15%). There were no study-related grade 4 or 5 toxicities. Possible immune-related adverse events included 4 cases of hypothyroidism, 2 cases of colitis and 1 case each of pneumonitis, erythema multiforme and seronegative arthritis. After a median follow-up of 80 months (range, 15-134), 14 patients remain alive with 10 in continuous remission. Estimated 3-yr OS, DFS, NRM and CIR was 66%, 48%, 5% and 47% respectively. Transplant outcomes in auto-pembro recipients were compared to a contemporaneous cohort of consecutive AML patients receiving allo transplant in first or later CR (n=112) by Cox proportional hazards model, adjusting for significant demographic and risk variables. Compared with allo recipients, auto-pembro recipients were less likely to be non-Hispanic white (45% vs. 69%) and male (35% vs. 59%) but were otherwise similar in regard to baseline patient and disease characteristics. In multivariable analysis, OS and DFS were significantly impacted by age, ELN risk and year of transplant. In contrast, transplant type (auto-pembro vs. allo) had no effect on OS (HR 0.58, p=0.24) or DFS (HR 1.19, p=0.65). Adjusted 3-yr OS and DFS was 76% vs. 66% and 54% vs. 62% respectively (see figure). Although relapse was significant higher following auto-pembro (HR 2.38, p=0.031), it was offset by a corresponding decrease in NRM risk. In conclusion, auto-pembro is a safe and effective consolidation therapy for non-favorable AML patients who are ineligible for allo transplant, with comparable adjusted survival outcomes. Correlative studies are planned to analyze anti-leukemic effect of post-transplant PD-1 blockade following autologous transplant in AML patients. Given these promising results in non-favorable-risk AML patients lacking an allogeneic transplant consolidation option, confirmation in a larger multicenter prospective study is warranted.
Disclosures
Solh:ADC Therapeutics: Research Funding; Partner Therapeutics: Research Funding.
OffLabel Disclosure:
Pembrolizumab is being utilized off-label for consolidation therapy post-autologous transplant for patients with acute myeloid leukemia
Author notes
Asterisk with author names denotes non-ASH members.